Novel Biologics & Biosimilars
Paras Biopharmaceuticals' Novel Biologics and Biosimilar Pipeline & Technologies
Novel Biologics and Biosimilars Pipeline
Paras Biopharma Novel Biologics
Paras Biopharma Novel Biologics approach and portfolio pipeline comprises of Biologics for Rare & Orphan Diseases and Novel applications in Multiple Myeloma, Respiratory, Gout, Pulmonary Arterial Hypertension, Osteoarthritis, and Knee Fibrosis.
| Multiple Myeloma |
Respiratory |
Gout |
Pulmonary Arterial Hypertension |
Osteoarthritis |
Knee Fibrosis |
Paras Biopharma Biosimilars
Paras Biopharma Biosimilar approach and portfolio pipeline comprises of unique biosimilars that have niche indications, low competition, high value and growing market opportunity, and low risk for development. Product pipeline includes biosimilar candidates of Oncology, Osteoporosis, Rheumatoid Arthritis, and Metabolic Diseases.
| Oncology |
Osteoporosis |
Rheumatoid Arthritis |
Metabolic Diseases |
Biosimilar Overview
Paras Biopharmaceuticals is committed to innovation of high-quality products and technologies. The Company has developed robust biosimilars development technologies for the following medical areas:
Oncology products for: multiple myeloma, platelet disorder, and tumor lysis syndrome.
Biosimilar Technologies/Product Pipeline
| Paras Biopharma Product / Biosimilars |
Target Disease |
Brand Name(INN Name) |
Description |
Technology Platform |
| PB (Osteo)-1010 |
Osteoporosis |
Terishield™ (Teriparatide) |
Biosimilar candidate to Forteo® |
Diabrid™Technology / NobleCleav™Technology |
| PB (RA)-2010 |
Rheumatoid Arthritis |
IL-KTM™(Anakinra) |
Biosimilar candidate to Kineret® |
High Expression Technology |
| PB (MDT)-3010 |
Type I and II Diabetes |
Asplin™(Insulin Aspart) |
Biosimilar candidate to Novolog® |
Diabrid Technology / NobleCleav™Technology |
| PB (CT)-4020 |
Oncology (Platelet Disorders) |
Romi-Multiplat™ (Romiplostim) |
Biosimilar candidate to Nplate® |
Biomultifold™ Technology |
| PB (CT)-4030 |
Oncology (Tumor Lysis Syndrome) |
Raspa™ (Rasburicase) |
Biosimilar candidate to Elitek® |
High Expression Technology |
Disclaimer
Products under patents are part of our research projects. These products may be offered only in those countries where patents have expired.
For the latest status, please contact Paras Biopharmaceuticals Finland Oy.
Forteo®, Kineret®, Elitek®, Nplate®, and Novolog® are registered trademarks of/marketed by Eli Lilly, Sobi/Amgen, Amgen, Sanofi and Novo Nordisk respectively. *Source GlobalData
| Paras Biopharma Product / Biosimilars |
Target Disease |
Brand Name(INN Name) |
Description |
Development Stage |
| PB (RA)-2020 |
Rheumatoid Arthritis |
Ceras-peg™(certolizumab) |
Biosimilar candidate to Cimzia® |
Under development |
| PB (CT)-4040 |
Multiple Novel Applications |
IL-MTM™ |
Biobetter candidate to IL-1 RA |
Under development |
| PB (CT)-4010 |
Oncology (Multiple Myeloma) |
IL-BTM™ |
Biobetter candidate to IL-1 RA |
Under development |
| PB (OA)-5010 |
Osteoarthritis |
IL-OTM™ |
Osteoarthritis patch of IL-1 RA |
Under development |
Disclaimer
Products under patents are part of our research projects. These products may be offered for further development only in those countries where patents have expired. For the latest status, please contact Paras Biopharmaceuticals Finland Oy.
Cimzia® is a registered trademarks of/marketed by UCB. *Source GlobalData
Unique Features
 Paras Biopharmaceuticals innovative technology platform, along-with other associated process technologies, enables a very high and economical way to develop biosimilars for scale-up production.
Paras Biopharmaceuticals innovative technology platform, along-with other associated process technologies, enables a very high and economical way to develop biosimilars for scale-up production.
The Paras Biopharmaceuticals’ Team has successfully developed a multiple biosimilar in its pipeline for “Rare & Orphan diseases” which are now available in the company’s portfolio for partnership.
Biosimilars Pipeline & Technologies
Development of Recombinant Expression Systems & Process Technologies for Biologics & Biosimilars:
Paras Biopharmaceuticals is actively engaged in research & development and is offering partnership for further development (including clinical development) on the following biosimilars:
- Recombinant Teriparatide (Forteo®Biosimilar).
- Recombinant IL1-RA / Anakinra (Kineret®Biosimilar).
- Analog Insulin Aspart.
- Recombinant Romiplostim (Nplate®Biosimilar).
- Further biologics are in the pipeline.
Due to its own technologies, Paras Biopharmaceuticals has achieved efficient and cost-effective ways which
would help in the efficient production of some high value biosimilars. This development is based on Paras
Biopharmaceuticals' proprietary technology platform which includes Diabrid®, Noblecleav®, Biomultifold® &
Cytofold StructQuant® technologies.
Forteo®, Kineret® and Nplate® are registered trademarks of/marketed by Eli Lilly, Sobi Sweden and Amgen USA respectively * Source GlobalData
Biosimilars Technologies/Product Pipeline
| Paras Biopharmaceuticals Biosimilar pipeline |
Terishield™ |
IL-KTM™ |
Asplin™ |
| Reference Product |
Forteo® |
Kineret® |
Novolog® |
| INN |
Teriparatide |
Anakinra |
Insulin Aspart |
| Major Approved indications |
Postmenopausal Osteoporosis |
Rheumatoid Arthritis, CAPS#, DIRA* |
Diabetes Mellitus |
| 2020 Global sales* |
$ 1076m |
$ 220m |
$ 4385m |
| Development stage |
Scale-up production and comparison |
Scale-up production and comparison |
Pre-clinical (high expression achieved) |
Disclaimer
Products under patents are part of our research projects. These products may be offered for further development only in those countries where patents have expired. For the latest status, please contact Paras Biopharmaceuticals Finland Oy.
Forteo®, Kineret®, and Novolog® are registered trademarks of/marketed by Eli Lilly, Sobi/Amgen, and Novo Nordisk respectively * Source Globaldata
| Paras Biopharmaceuticals Biosimilar pipeline |
Romi-MultiPlat™ |
Raspa™ |
Ceras-Peg™ |
| Reference Product |
Nplate® |
Elitek® |
Cimzia® |
| INN |
Romiplostim |
Rasburicase |
Certolizumab-pegol |
| Major Approved indications |
Immune Thrombocytopenia |
Mgmt. of plasma uric acid levels in oncology patients |
TNF blocker indicated for Rheumatoid Arthritis and others |
| 2020 Global sales* |
$ 920m |
$ 170m |
$ 210m |
| 2025 Sales estimates* |
$ 964m |
$ 314m |
$ 1847m |
| Development stage |
Scale-up production & biosimilarity/ Pre-clinical |
Under development (high expression achieved) |
Under development |
Disclaimer
Products under patents are part of our research projects. These products may be offered for further development only in those countries where patents have expired. For the latest status, please contact Paras Biopharmaceuticals Finland Oy.
Nplate®, Elitek®, and Cimzia® are registered trademarks of/marketed by Amgen, Sanofi, and UCB respectively * Source Globaldata
PB (Osteo)-1010 (Biosimilar candidate to Forteo®)
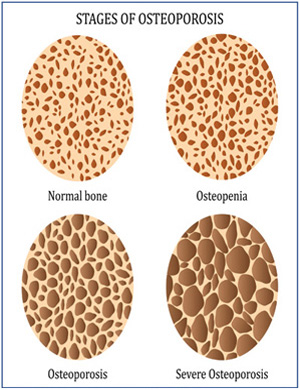 Osteoporosis
Osteoporosis
Brand Name:
Terishield™ |
INN:
Teriparatide |
Reference Product:
Forteo® |
- PB Osteo-1010 is a recombinant form of the parathyroid hormone whose molecular weight of the final Drug Substance is 4117 g/mol.
- PB-osteo-1010 has been developed for use in post-menopausal women with Osteoporosis possessing a high risk of fracture or with a history of Osteoporosis fracture.
- The National Osteoporosis Foundation estimated that in 2020 61 million people experienced the effects of Osteoporosis or Osteopenia. Women are affected in greater numbers as they have a lower peak bone density.
- Paras Biopharmaceuticals has successfully achieved a unique and novel process to obtain high expression.
Forteo® is a registered trademark of/marketed by Eli Lilly.
PB (Osteo)-1010 (Osteoporosis)
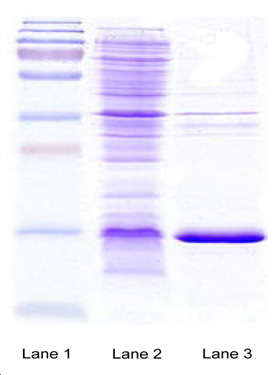 Product Status:
Product Status:
- SDS-PAGE high Expressions
- Separation and purification of Diabrid™ achieved
- Confirmation through mass spectrometry
Technology Status
- Soon ready to be out-licensed with scale-up production established.
- Recombinant state E. Coli clone exhibit high expressionof the osteoporosis product (EM@39000-Paras Rights Reserved).
- SDA-PAGE-Gel image showing marker (lane 1), homogenization broth (lane 2) and separation of product in Diabrids (lane 3).
- Multiple batches carried out at small & large scales.
- Processes optimized at scale-up level.
- Biocomparability methods established.
- Extensive product characterization done.
- Biosimilarity established.
PB (RA)-2010 (Biosimilar candidate to Kineret®)
 Rheumatoid Arthritis
Rheumatoid Arthritis
Brand Name:
IL-KTM™ |
INN:
Anakinra |
Reference Product:
Kineret® |
- PB-RA-2010 is (originator soon to be off patent) a recombinant human Interleukin-I receptor-antagonist protein.
- PB-RA-2010 is a biosimilar candidate molecule that works by blocking the biological activity of the IL-I receptor. IL-I is a key mediator of inflammation and a driver of auto inflammatory diseases in both adults and children.
- PB-RA-2010 is a recombinant non-glycosylated version of IL-IRA produced in genetically modified E. Coli using recombinant DNA technology. It consists of 153 a.a.and possesses a molecular mass of 17.3 KDa.
- Paras Biopharmaceuticals Finland Oy has successfully achieved a very high expression of the Biosimilar Rheumatoid Arthritis candidate.
Kineret® is a registered trademark of/marketed by Sobi/Amgen.
PB (RA)-2010 (Rheumatoid Arthritis)
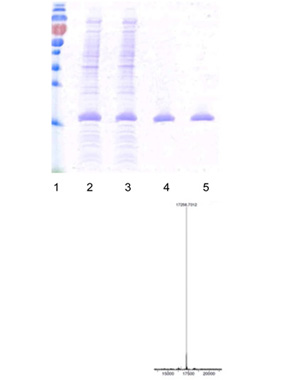 Product Status:
Product Status:
- SDS-PAGE high Expressions
- Separation and purification of Diabrid™ achieved
- Confirmation through mass spectrometry
- Very high yield of product achieved –multiple grams per liter yield of purified material
Technology Status
- Technology ready to be out-licensed for scale-up production.
- Recombinant state E. Coli clone exhibit expression of the Rheumatoid product (EM@39000-Paras Rights Reserved).
- SDA-PAGE-Gel image showing marker (lane 1), homogenization broth (lane 2) and separation of product (lane 3). Lanes 4 & 5 show the purified product.
- Mass spectrometry (MS) data shows confirmation of the correct product.
- Multiple batches carried out at small & large scales.
- Processes optimized at scale-up level
- Extensive product characterization done
- Biocomparability methods established
- Biosimilarity established
PB (MDT)-3010 (Biosimilar candidate to Novolog®)
 Diabetes (Metabolic Disorders)
Diabetes (Metabolic Disorders)
Brand Name:
Inspart™ |
INN:
Insulin Aspart |
Reference Product:
Novolog® |
- Paras Biopharmaceuticals Finland Oy has successfully developed a high expression and have Analog Insulin Biosimilar production clones.
- Rapid acting analog "Aspart" is produced with Paras Diabrid™ Technology.
- The process for the production of insulin "Aspart" is currently being prepared for worldwide PCT patent submission. These technological innovations by Paras Biopharmaceuticals reduces the costs of industrial production of Insulin "Aspart" achieving higher economic advantages.
Novolog® is a registered trademark of/marketed by Novo Nordisk.
PB (MDT)-3010 (Diabetes)
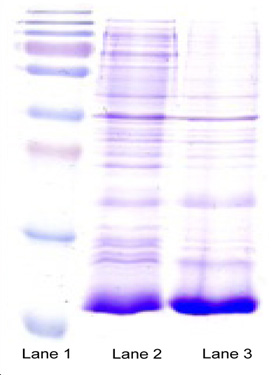 Product Status:
Product Status:
- SDS-PAGE high Expressions
- Mass spectrometry: Confirmation of N-& C-Terminal, and AA sequence
Technology Status
- Soon to be available for licensing within scale-up production
- Recombinant state E. Coli / S. cerevisae clone exhibits expressions of the metabolic disorder treatment (PB (MDT)-3010) (EM@39000 -Paras Rights Reserved)
- SDA-PAGE-Gel image showing marker (lane 1), homogenization broth (lane 2) and separation of metabolic disorder treatment product (lane 3)
- Multiple batches carried out at small & large scales
- Processes optimized at scale-up level
- Biocomparability methods established
- Extensive product characterization done
- Biosimilarity established
Advantages of Biosimilars
| Salient Features |
Small Molecule |
Biosimilars (follow-on biologics) |
Novel Biologics |
| Market entry barriers |
Low |
High -(Technology and scientific knowhow available with Paras Biopharmaceuticals) |
High |
| Product development costs |
Low |
Medium |
Very High |
| Price advantage |
Low -(high discount on innovator molecule price) |
High -(low discount on reference product price) |
N.A. |
| USPs |
First to market and low price |
Low competition -(Niche product dev. capabilities by Paras Biopharmaceuticals) |
Differentiated therapies; |
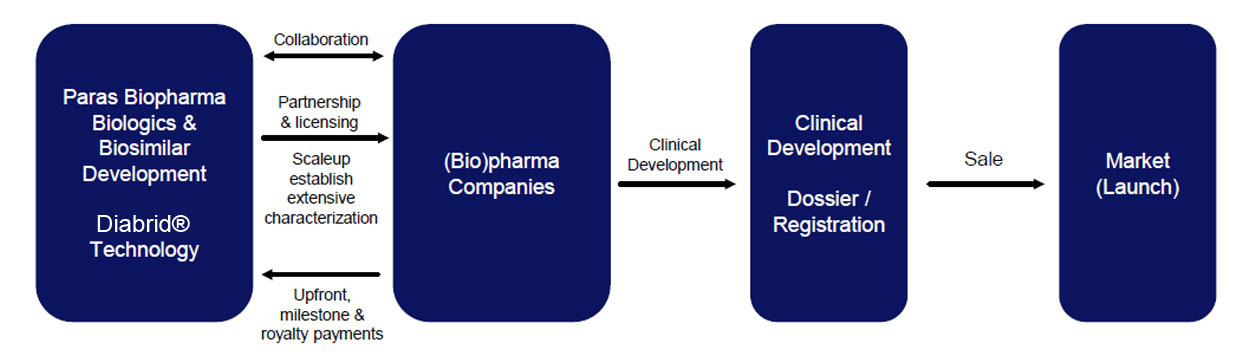
Paras Biopharmaceuticals Business Model
Paras Biopharmaceuticals has generated an authentic and well-characterized biosimilars production process using its Diabrid®, NobleCleav® & Biomultifold® technologies
Company’s business model works on the following features:
1. In-house biosimilar development has established an economical production process & extensive characterization.
2. Collaboration with (bio)pharmaceutical companies for clinical development / commercialization.
Biosimilar Market Insights
- As per one estimates, global biosimilars market is expected to reach US$ 35,7 bn by 2025 from US$ 11,8 bn in 2020 at a CAGR of 24.7%.
- Potential savings in use of biosimilars have been anticipated to cross US$ 100 bn by 2020.
- Recombinant non-glycosylated proteins accumulated a significant revenue share for the biosimilars market in 2018 and the value is likely to grow at a 26.1% CAGR through 2025.
- From 2015 to 2023, approx. US$ 122,7 worth of biologics be off–patent opening new and major opportunities.
- European Authorities have taken a lead on approval of Biosimilars.
- In 2012 or so, 16 Biosimilars were approved in Europe. The recommendations for approval of biosimilars has reached 77 by Feb 2021.
- New Law in USA-Biologics Price Competition and Innovation Act (enacted in 2010). Based on this law, the US-FDA has the authority to approve similar versions of biologic products (“biosimilars”).
- US-FDA issued draft guidelines for biosimilars approval (2012).
Osteoporosis Market Insights
- Osteoporosis is often called the "silent disease" because bone loss occurs without symptoms. It is characterized by low bone mass and deterioration of bone tissue, leading to bone fragility and a consequent increase in risk of fracture.
- As per International Osteoporosis Foundation census, more than 200 million people in the world are affected by osteoporosis, of which about 80% are women.
- Osteoporosis represents an implacable epidemic growing rapidly in regions of North America, Europe, and Japan.
- Osteoporosis prevalence is expected to increase due to the general aging population. In addition to increasing awareness, diagnosis and treatment.
- By 2020 or so, the total male and female osteoporosis population was forecast to increase by 16.0% to reach 188 million patients across the seven major markets alone.
- There are four Osteoporosis Drug Class (Bisphosphonate, SERM, PTH and Calcitonin) market.
- Top Osteoporosis Drug Brands are –FORTEO, PROLIA, Fosamax, Actonel, Reclast, Boniva, Evista, Viviant/Conbrizaand Miacalcin.
- A rapid uptake of Forteo/Forsteoand Prolia make these two products the most significant market drivers of osteoporosis therapeutics market.
Diabetes & Insulin Market Insights
- By 2030, there will be 436 million diabetics worldwide.
- Diabetes is a chronic disease leading to life-long use of medicines.
- Diabetes markets to grow as prevalence of over-weight and obesity is increasing rapidly.
- Diabetes affects more than 32 million Americans today. These numbers are expected to grow to 53 million in 2025.
- The annual budgets of medical and indirect society costs are expected to rise from 300 Billion USD to 515 Billion USD in 2025.
- Demand for Diabetes products are growing as life expectancy is also growing.
- By 2050, life expectancy is expected to increase and reach over 80 years. This will further fuel growth and needs of diabetes products globally.
- Insulin markets are growing with sustained double-digit growth.
- Demand of modern Insulins is growing more rapidly, and modern Insulins are replacing normal Insulin and animal Insulins.
- Total Sales of Diabetes products are expected to cross sales of 32 billion dollars a year.
- A major opportunity lies in the emerging markets for insulin, especially in the BRIC nations.



 Paras Biopharmaceuticals innovative technology platform, along-with other associated process technologies, enables a very high and economical way to develop biosimilars for scale-up production.
Paras Biopharmaceuticals innovative technology platform, along-with other associated process technologies, enables a very high and economical way to develop biosimilars for scale-up production. Osteoporosis
Osteoporosis Product Status:
Product Status: Rheumatoid Arthritis
Rheumatoid Arthritis Product Status:
Product Status: Diabetes (Metabolic Disorders)
Diabetes (Metabolic Disorders) Product Status:
Product Status: