






















 Paras Biopharmaceuticals has acquired and fully own a microbial production facility in Finland which was constructed in 2003 as a contract manufacturing site for microbial production of recombinant products in microbial strains. With a total floor area of 25,000 ft2 and a classified cleanroom of 4,300 ft2, other features include media and buffer preparation, live area (fermentation and harvest & extraction), purification suite (three separate rooms, incl. +4°C cold room), final filtration and freeze-drying. Production area in A/B, C and D classes including Operators’ rooms in key areas. Our team has strong experience in carrying out microbial biologics production.
Paras Biopharmaceuticals has acquired and fully own a microbial production facility in Finland which was constructed in 2003 as a contract manufacturing site for microbial production of recombinant products in microbial strains. With a total floor area of 25,000 ft2 and a classified cleanroom of 4,300 ft2, other features include media and buffer preparation, live area (fermentation and harvest & extraction), purification suite (three separate rooms, incl. +4°C cold room), final filtration and freeze-drying. Production area in A/B, C and D classes including Operators’ rooms in key areas. Our team has strong experience in carrying out microbial biologics production.
Timelines
Manufacturing site established: 2001 to 2003
Contract manufacturing: 2003 to 2009
Internal R&D: 2009 - 2012
Paras Biopharmaceuticals’ Technology development: 2012 onwards
Contact manufacturing: Open
 Paras Biopharmaceuticals’ process development capabilities stem from solid scientific knowledge and an excellent infrastructure. We offer a microbial platform at our production facility in Oulu, Finland where the laboratory is fully configurable to specific client needs.
Paras Biopharmaceuticals’ process development capabilities stem from solid scientific knowledge and an excellent infrastructure. We offer a microbial platform at our production facility in Oulu, Finland where the laboratory is fully configurable to specific client needs.
Process Development:
Our expertise in the development of robust bioprocesses allows the client’s project to be efficiently and effectively integrated into a commercially viable manufacturing process.
Our scales of operation are:
Fermenters: 10L, 150L and 750L (Sartorius) stainless steel bioreactors
Disposable, scalable bioreactor technologies
Process Optimization:
Fermentation processes are optimized to maximize expression and enhance process reproducibility.
 Paras Biopharmceuticals’ core expertise covers all aspect of downstream development. This includes the purification team who are able to automate most of the purification process, whilst our scientists have extensive experience in process optimization to provide the highest product recovery at a small scale. We also provide a full understanding of the scale up parameters to reach a robust production scale process to meet our clients’ needs and high standards.
Paras Biopharmceuticals’ core expertise covers all aspect of downstream development. This includes the purification team who are able to automate most of the purification process, whilst our scientists have extensive experience in process optimization to provide the highest product recovery at a small scale. We also provide a full understanding of the scale up parameters to reach a robust production scale process to meet our clients’ needs and high standards.
Process Development:
 Paras Biopharmaceuticals provides a complete purification solution including:
Paras Biopharmaceuticals provides a complete purification solution including:
Primary separation
Centrifugation / cell disruption
Ultra- and diafiltration using hallow fibre and cassette filters
Liquid chromatography-based purification utilizing leading chromatography systems:
ÄKTA Pilot (GE Healthcare), ÄKTA Process, ÄKTA Explorer 100
AxiChrom 300, AxiChrom 200, AxiChrom 150, AxiChrom 100, AxiChrom 70, AxiChrom 50. (15 columns in total)
Our competence include chromatographic techniques such as ion exchange, hydrophobic interaction, expanded bed, affinity and gel filtration.
Process optimization:
Besides our focus on developing robust bioprocesses with high yield and purity, we employ extensive in-process analytical testing to identify and control process-related impurities. This also includes the monitoring of post-translational modifications of our products such as glycosylation. We deliver a full bio-comparability testing profile for your product.
FACILITY

MEDIA & BUFFER PREPARATION
Constructed in 2003 as a contract manufacturing site for microbial production of recombinant products in microbial strains.
Facility designed by Kvaerner UK (now Aker Solutions) and Elomatic Finland.

FREEZE DRYING / LYOPHILISATION STUDIES
HIGH-PRESSURE LIQUID CHROMATOGRAPHY HPLC-BASED PURIFICATION AT LARGE SCALE

AXICHROM COLUMNS
Our competence include chromatographic techniques such as ion exchange, hydrophobic interaction, expanded bed, affinity and gel filtration.

High-pressure Liquid chromatography
HPLC-based purification
Paras Biopharmaceuticals Finland Oy (Paras) offers optimized scale-up biologic and manufacturing technologies whilst specialising in microbial fermentation based recombinant productions. Paras’ aim is the commercial production of biologics APIs (Active Pharmaceutical Ingredients) with our core competence geared towards scale-up and GMP manufacturing. In addition, through our partnering network, we offer integrated services for final fill and finish.
Production Facility Technical Features-
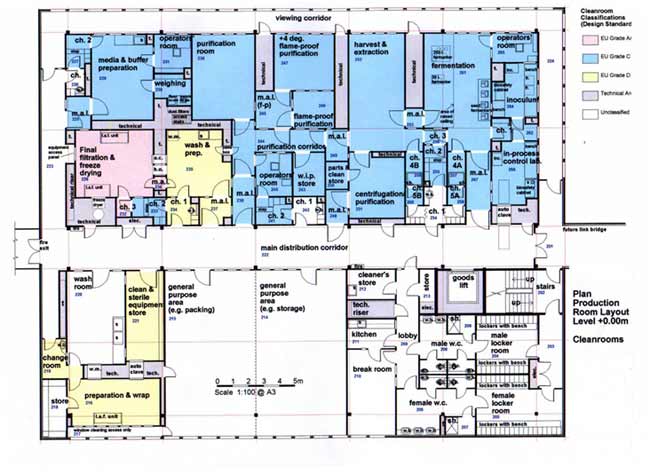
Total floor area 2300 m2
Classified cleanroom 400 m2
Production area in A/B, C and D classes Operators’ rooms in key areas,
 Paras process development capabilities stem from solid scientific knowledge and an excellent laboratory infrastructure at our facilities in Oulu, Finland. Built in 2001, it includes modern labs which are fully equipped with the most advanced analytical equipment, Bioreactors and protein purification equipment (AKTA Exploter 100, AKTA pilot and Axichrom columns etc).
Paras process development capabilities stem from solid scientific knowledge and an excellent laboratory infrastructure at our facilities in Oulu, Finland. Built in 2001, it includes modern labs which are fully equipped with the most advanced analytical equipment, Bioreactors and protein purification equipment (AKTA Exploter 100, AKTA pilot and Axichrom columns etc).
Production facilities in Oulu, Finland are fully equipped with modern analytical equipment:
|
|
  |
Production facilities possess support systems in four key areas: air handling, waste decontamination, alarm management and validation.
|
|
  |
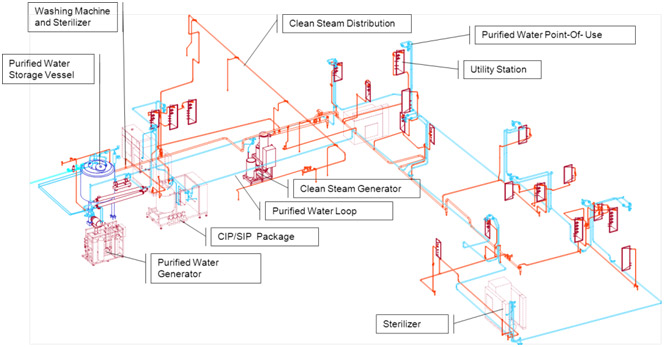
Production facilities are fully equipped with modern process utilities, HVAC system and SCADA controls allow flexible production capabilities for biologic-products. Together with state-of-the art clean room technology and production equipment, a range of capacities in fermentation and downstream processes are available.
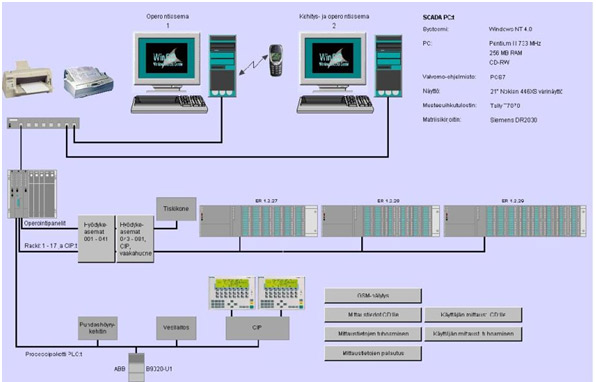

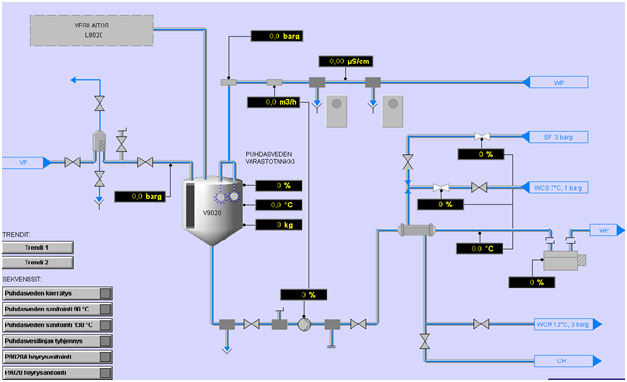
Validated SCADA-system controls
Paras Biopharmaceuticals
Finland Oy,
Kiviharjunlenkki 10, OULU,
FI-90220 Finland
P: +358 442709462
Dr Ashesh Kumar
E: kumar.ashesh@parasbiopharma.com
P: +358 (0) 400207380
Dr Mark Jackson
E: mark.jackson@parasbiopharma.com
P:+358 (0) 442905993
Skype: Paras.Finland
Copyright © Paras Biopharmaceuticals Finland Oy 2025. All Rights Reserved.
Website Design and Development by Sterco Digitex
This website uses cookies; by continuing to use this page, you consent to their use. I Understand About Cookies.
