 PB Osteo-1010 is a biosimilar: Soon to be off patent. Osteoporosis product Biosimilar Technology is product Biosimilar Technology.
PB Osteo-1010 is a biosimilar: Soon to be off patent. Osteoporosis product Biosimilar Technology is product Biosimilar Technology.
PB-osteo-1010 is a recombinant form of the parathyroid hormone whose molecular weight of the final API is 4117 g/mol. PB-osteo-1010 has been developed for use in post-menopausal women with Osteoporosis possessing a high risk of fracture or with a history of Osteoporosis fracture.
Increased bone mass in men with Osteoporosis and patients with multiple risk of fracture have also been taken into account during development.
The National Osteoporosis Foundation has estimated that 44 million people are currently experiencing the effect of Osteoporosis or Osteopenia. By 2020, this will have risen to more than 61 million people. Women are affected in greater numbers as they have a lower peak bone density.
Paras Biopharmaceuticals Finland Oy has successfully achieved a unique and noble process to obtain high expression.
| Product status |
|
|
| Technology status | 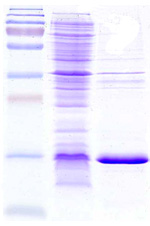 |
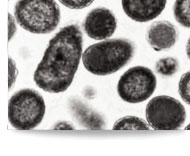
Soon ready to be out licensed for scale up production. Recombinant stable E. Coli Clone exhibit deposits of the Osteoporosis product inside the cells (EM@39000-Paras Rights Reserved). SDS-PAGE-Gel image (left) showing marker (lane1), homogenization broth (lane2) and separation of product in Diabrids (lane3). |
Osteoporosis Rheumatoid Arthritis Diabetes (Metabolic Disorder) AMD (Biosimilar Lucentis Ranibizumab) Oncology Market Insights
This website uses cookies; by continuing to use this page, you consent to their use. I Understand About Cookies.