Rheumatoid Arthritis - API Product PB (RA) - 2010 & Biosimilars Technology
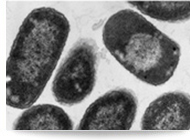 PB-RA-2010 is soon to be off patent involving biosimilars API / biosimilars technology for the production of recombinant human Interleukin-I receptor-antagonist protein. The product is used for the treatment of Rheumatoid Arthritis (RA). PB-RA-2010 is a biosimilars molecule that works as part of a group of recombinant products by blocking the biological activity of the IL-I receptor. IL-I is a key mediator of inflammation and a driver of auto inflammatory diseases in both adults and children.
PB-RA-2010 is soon to be off patent involving biosimilars API / biosimilars technology for the production of recombinant human Interleukin-I receptor-antagonist protein. The product is used for the treatment of Rheumatoid Arthritis (RA). PB-RA-2010 is a biosimilars molecule that works as part of a group of recombinant products by blocking the biological activity of the IL-I receptor. IL-I is a key mediator of inflammation and a driver of auto inflammatory diseases in both adults and children.
 PB-RA-2010 is a recombinant non-glycosylated version of IL-IRA produced in genetically modified E. Coli using recombinant DNA technology. It consists of 153 a.a. and possesses a molecular mass of 17.3 KDa. As a biosimilar, it is an approved therapy for RA.
PB-RA-2010 is a recombinant non-glycosylated version of IL-IRA produced in genetically modified E. Coli using recombinant DNA technology. It consists of 153 a.a. and possesses a molecular mass of 17.3 KDa. As a biosimilar, it is an approved therapy for RA.
Paras Biopharmaceuticals Finland Oy has successfully achieved a very high expression of the Biosimilar Rheumatoid Arthritis product.
| Product status | SDS-PAGE High expressions
» Separation and purification of Diabrid achieved.
"Very high yield of product achieved - multiple grams per liter yield of purified material." » Confirmation through mass spectrometry. |
|
| Technology status | 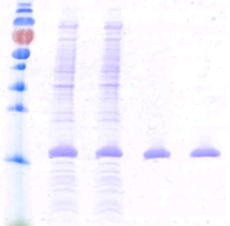 |
Technology ready to be out licensed for scale up production. Recombinant stable E. Coli Clone exhibit deposits of the Rheumatoid product inside the cells (EM@39000-Paras Rights Reserved). SDS-PAGE-Gel image (left) showing marker (lane1), homogenization broth (lane2) and separation of product (lane3). Lane 4 and 5 show the purified product. |
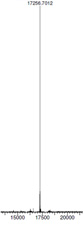 |
Mass spectrometry (MS) data ( left side) shows confirmation of the correct product. Very high yield of product achieved - multiple grams per liter yield of purified material. |
|
Paras Biopharmaceuticals
Finland Oy,
Kiviharjunlenkki 10, OULU,
FI-90220 Finland
P: +358 442709462
Dr Ashesh Kumar
E: kumar.ashesh@parasbiopharma.com
P: +358 (0) 400207380
Dr Mark Jackson
E: mark.jackson@parasbiopharma.com
P:+358 (0) 442905993
Skype: Paras.Finland
This website uses cookies; by continuing to use this page, you consent to their use. I Understand About Cookies.